A Corrosive Stomach Environment
Your stomach acid has a pH of around 2.0. That’s the same pH
as hydrochloric acid (HCL). Put a few drops of HCL on your kitchen table and it
won’t be long before you have a hole in the table.
The reason our stomachs need that low a pH is beyond the scope of this simple post, but I hope you will wonder WHY that extremely corrosive acidic environment in our stomach doesn’t dissolve our stomach.
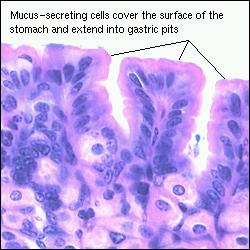
One reason has to do with the mucous cells lining our stomach wall. They serve as a protective coating to prevent that from occurring.
So, think about this: It is either an astounding bit of luck in the course of our evolutionary development that the mucous cells exist in our stomach . . . .
The reason our stomachs need that low a pH is beyond the scope of this simple post, but I hope you will wonder WHY that extremely corrosive acidic environment in our stomach doesn’t dissolve our stomach.
One reason has to do with the mucous cells lining our stomach wall. They serve as a protective coating to prevent that from occurring.
So, think about this: It is either an astounding bit of luck in the course of our evolutionary development that the mucous cells exist in our stomach . . . .
Or those cells were specifically designed by our Creator to
protect the stomach from being dissolved by the acid.


Comments
Post a Comment